Learn more about
Brief Product Outline and Illustration
Questions about GONAL-f® Pen ?
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
Brief Product Outline and Illustration
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
Before GONAL-f treatment, be sure to ask your doctor…
Every treatment is individualized. Your treatment has been carefully designed for you by your doctor. Over the course of your treatment, doses may range between 75 to 450 IU depending on your specific medical condition and your response to the medicine.
IU is International Units
Do not use GONAL-f if you:
GONAL-f is often used with other medicines to stimulate ovulation (e.g., hCG and LH), which may help the maturation of follicles that contain the eggs. If a GnRH agonist or antagonist medicine is also given along with your GONAL-f in your treatment program, you may be told by your doctor to change your dose of GONAL-f over the course of your treatment.
No other significant interactions with other medicines have been reported.
Fertility drugs are safe to take with close monitoring by your doctor. As with all medication, there is a potential for side effects; the following have been reported with the use of GONAL-f during clinical trials and post-market use:
Common and Very Common: may affect 1 to 10 users in 100
Uncommon, Rare and Very Rare: may affect less than 1 to 10 users in 1,000
The greatest concern your doctor will have is OHSS. To avoid this, your doctor will carefully monitor your response to GONAL-f. Ovarian enlargement, sometimes accompanied by abdominal bloating and pain, may occur in about 20% of patients taking gonadotropins. This is generally reversed with cessation of treatment and severe life-threatening cases are rare.
A causal relationship between treatment with fertility drugs and ovarian cancer has not been established.
The following medical events have been reported subsequent to pregnancies resulting from gonadotropin therapy in controlled clinical trials: spontaneous abortion, tubal pregnancy, premature labour, postpartum fever and congenital abnormalities.
None of these events were thought to be drug-related; incidence is not more than that found in the general population.
This is not a complete list of side effects. If you experience any unusual symptoms or side effects while taking GONAL-f, report them to your doctor or pharmacist immediately. You should discuss the possibility of side effects with your doctor before you begin treatment.
General
To help avoid side effects and ensure proper use, talk to your doctor or pharmacist before you take GONAL-f. Your fertility should be evaluated before the treatment is started by a doctor experienced in treating fertility disorders.
Porphyria
If you have porphyria or a family history of porphyria, GONAL-f may increase the risk of an acute attack. Tell your doctor immediately if:
In case of the above events your doctor may recommend that you stop treatment.
Overstimulation of the Ovary During FSH Therapy
Ovarian Enlargement
Ovarian Hyperstimulation Syndrome (OHSS)
Breathing and Blood Clotting
Reproductive Issues
GONAL-f is available in 3 pre-filled pen formats:
No, this product does not contain latex.
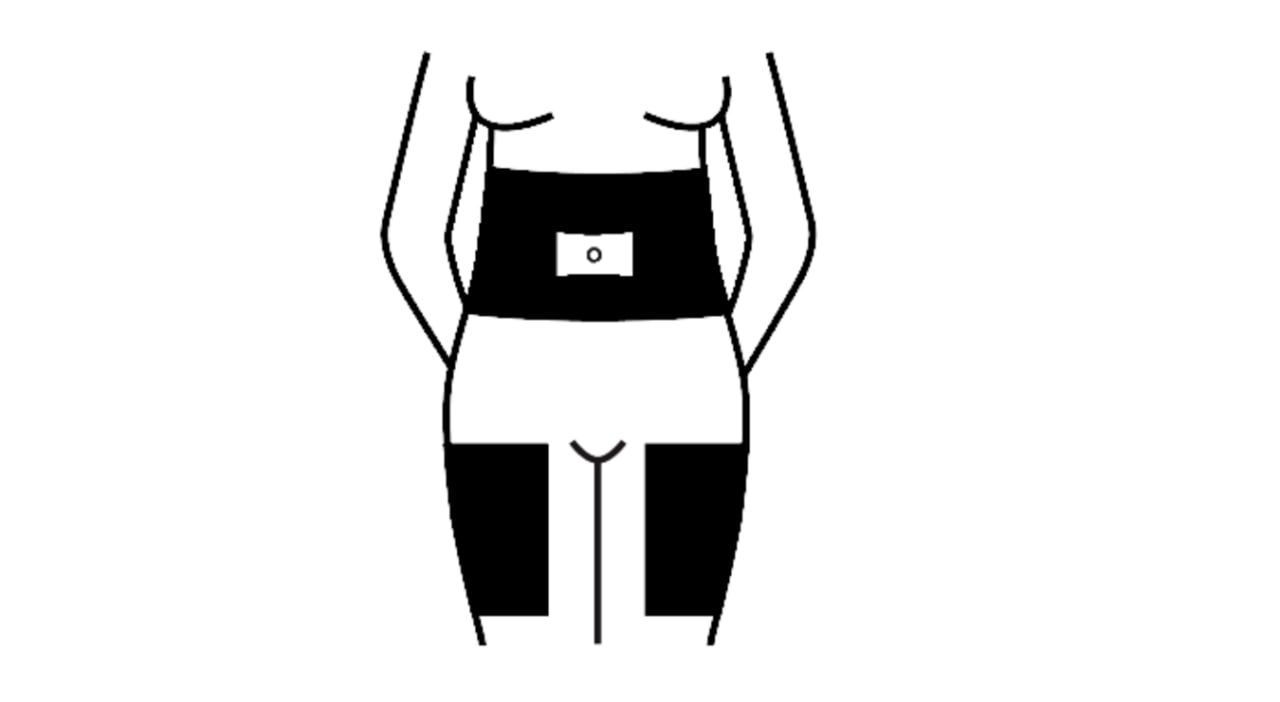
The most easily accessible areas for subcutaneous injection are the abdomen and thighs. The absorption of GONAL-f is the same regardless of the injection site selected. You may find the injection is more comfortable if you vary the site each time you inject GONAL-f.
Below is a diagram with shaded areas showing the recommended subcutaneous injection sites.
If you dialed the dose past your prescribed dose, simply turn the injection button back to the correct dose. To adjust the dose, you can turn the injection button either clockwise or counter-clockwise. After confirming the correct dose from the display window, you can proceed with the injection.
If you have accidentally injected too much GONAL-f, do not panic. Simply contact your physician or healthcare professional for further instructions.
IMPORTANT: The maximum dose that can be dialed is 450 IU for the 900 & 450 IU Pen & 300 IU for the 300 IU Pen.
Do not take a double dose to make up for any doses you have missed. Contact your doctor for advice if you forget to take a dose of GONAL-f.
When you cannot push the injection button down, it means your pen is empty and it is unable to provide you with your desired dose; you may still see a small amount of medication remaining in the pen – this is normal.
If the dose display shows a number higher than 0 after your injection, this indicates that the pen is empty and that the complete dose has not been given. If this happens, the number shown in the dose display indicates the missing amount of GONAL-f solution that is needed to complete your dose. This is the dose you need to inject using a new pen. To complete your dose, set the dose to the missing amount (displayed on the empty pen) that you have just finished, and proceed with the injection.
You should throw away the used needles safely in a sharps container. This container is usually provided by your clinic in a GONAL-f Pen Starter Kit.
You can dispose of your used GONAL-f Pens in an environmentally responsible manner by participating in the EMD Serono ecoProgram.
What is the ecoProgram?
EMD Serono's ecoProgram will help reduce landfill by turning used GONAL-f Pens to clean energy via the Waste-to-Energy (WtE) process. We care for patients, and our planet!
What is Waste-to-Energy (WtE)?
WtE is a process where waste (like used GONAL-f Pre-filled Pens) is burned in a controlled combustion chamber at high temperatures and reduced to 10% of its original volume (hence reducing landfill). The heat generated from the combustion chambers water is turned to steam and sent through a turbine that continuously generates electricity.
According to the US Energy Recovery Council, 87 WtE plants divert approximately 90,000 tons of waste each day from landfills, generating nearly 17 billion kilowatt hours of electricity per year. This is enough to power almost two million homes and represents nearly 20% of all non-hydro renewable electricity generation in the United States. To put this figure into context, it would take 7.8 million tons of coal to produce the same amount of electricity from a coal-fired power plant. (Source: www.energyrecoverycouncil.org)
How can I participate?
First, make sure your fertility clinic is participating in the GONAL-f ecoProgram. If not, ask them to join in the cause.
3 Easy steps:
1.Bring the used pens (without needles) back to your clinic.
2.Put pens in a reusable ecoProgram table-top container. When the container is full, simply empty the pens into the pre-labelled carton box provided.
3.The used pens will get sent to a WtE plant for processing to produce electricity.
GONAL-f® is a registered trademark of Merck KGaA, Darmstadt, Germany