Questions about Pergoveris?
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
Overview
PERGOVERIS® is a combination of GONAL-f® (follicle-stimulating hormone or FSH) and LUVERIS® (luteinizing hormone or LH) at a fixed dose of 150 IU FSH and 75 IU LH. It comes as a liquid formulation, in a pre-filled pen device, to be administered “subcutaneously” or “under the skin”.
Prior to therapy with PERGOVERIS, you should be informed of the duration of treatment and need for monitoring.
PERGOVERIS® is used to stimulate follicular development in hypogonadotropic hypogonadal women with severe LH (<1.2 IU/L) and FSH deficiency (≤5.0 IU/L), who are candidates for concurrent therapy with FSH and LH.
PERGOVERIS® should only be used under the strict supervision of a doctor.
Every treatment is individualized. Your treatment has been carefully designed for you by your doctor according to your specific needs. It is very important that you keep your appointments and follow your doctor’s instructions, particularly with regard to the amount and frequency of the medication you are taking.
A treatment regimen commences with the recommended dose of PERGOVERIS® containing 150 IU follitropin alfa (r-FSH) and 75 IU lutropin alfa (r-LH). According to your response, your doctor may increase your dose of follitropin alfa by 37.5-75 IU at 7- to 14-day intervals.
When the desired response has been obtained, you will receive a single injection of hCG 24-48 hours after your last injection of PERGOVERIS®. It is recommended that you have sexual intercourse the day of and the day following your hCG injection. Alternatively, intrauterine insemination or in vitro fertilization may be performed.
If an excessive response occurs, treatment should be stopped and hCG withheld. For the following cycle, your doctor may prescribe follitropin alfa at a lower dose than that used in the previous cycle.
It is recommended that you inject PERGOVERIS® at around the same time each day.
Do not use PERGOVERIS® if you have:
- Hypersensitivity to the active substances follitropin alfa and lutropin alfa
- Primary ovarian failure or anovulation with normal levels of FSH and LH
- Uncontrolled thyroid or adrenal dysfunction
- Tumours of the hypothalamus or pituitary gland
- Ovarian enlargement or cyst of unknown cause
- Gynecological bleeding of unknown cause
- Sex hormone dependent tumours of the reproductive tract and accessory organs
- Current pregnancy or lactation
- A condition which would make a normal pregnancy impossible, such as:
- Premature menopause
- Malformation of reproductive organs
- Specific tumours of the uterus, including severe uterine fibroids
Please tell your doctor or pharmacist if you are taking (or have recently taken) any other medicines, including over-the-counter products
PERGOVERIS® should not be administered with other medications in the same injection; it may be given at the same time as follitropin alfa in a separate injection, if prescribed by your doctor.
- Like all medications, PERGOVERIS® may cause side effects; some people commonly experience abdominal pain, pelvic pain, breast pain, constipation, painful menstruation, stomach or intestinal gas, headache, nausea, and reactions at the injection site (pain, redness, itching, bruising, swelling and/or irritation)
- There is a risk of developing ovarian hyperstimulation syndrome (OHSS) – the early warning signs are severe abdominal pain, nausea, vomiting and weight gain; OHSS can develop quickly, so if you experience any of these symptoms, contact your doctor immediately
- In serious, but rare cases, OHSS with clearly enlarged ovaries can include buildup of fluid in the abdomen or chest, as well as more serious complications (i.e., abnormal blood clotting); in rare cases, abnormal blood clotting can occur, independent of OHSS
- Ectopic pregnancy (i.e., embryo implanted outside the uterus) may occur, especially in women with history of prior disease/scarring of the Fallopian tubes
This is not a complete list of side effects. If you experience any of the conditions listed above or any unexpected side effect, please contact your doctor or pharmacist.
If you have porphyria, which is a group of inherited disorders (a disorder that may be passed on from parents to children), you should inform your doctor as the use of certain medications may trigger an attack of the illness. If you notice your skin becoming fragile and blisters easily (especially areas that are frequently exposed to sunlight) and/or you have stomach or limb pain, you should tell your doctor who may recommend that you stop treatment.
PERGOVERIS® treatment seldom gives rise to significant ovarian hyperstimulation syndrome (OHSS) unless the medicine used to induce final follicular maturation (containing human chorionic gonadotropin - hCG) is administered. In other patient populations, this treatment increases your risk of developing OHSS. It is therefore prudent to withhold administration of hCG in cases where OHSS is developing and not to have sexual intercourse. You should use barrier methods for at least four days.
If you are at risk of thromboembolic events (formation of a blood clot in vein or artery), because of your personal or family history, treatment with gonadotropins, like pregnancy itself, may further increase the risk. If you think you may have such a risk, please talk to your doctor.
In patients undergoing induction of ovulation, the incidence of a multiple pregnancy and births is increased compared with natural conception.
The frequency of miscarriages is higher than in the normal population, but similar to the rate found overall in women with fertility problems.
Women with a history of tubal disease are at a risk of ectopic pregnancy (pregnancy where the embryo is implanted outside the womb), whether the pregnancy is obtained by spontaneous conception or with fertility treatments.
There have been reports of tumours of the ovary and other reproductive organs, both benign and malignant, in women who have undergone multiple drug regimens for infertility treatment.
There have been isolated reports of non-serious allergic reactions to PERGOVERIS®. If you had this type of reaction to similar medicines, inform your doctor.
PERGOVERIS® is available in 3 pre-filled pen formats.
- 300 IU Pen (contains 300 IU r-hFSH/150 IU r-hLH in 0.48 mL)
- 450 IU Pen (contains 450 IU r-hFSH/225 IU r-hLH in 0.72 mL)
- 900 IU Pen (contains 900 IU r-hFSH/450 IU r-hLH in 1.44 mL)
“IU” is International Units
- Before first use, store at 2–8°C (refrigerator)
- Do not freeze
- Protect from light
- After first use, store at or below 25°C (room temperature), for a maximum of 28 days
- Do not use after expiry date
- Do not use if you notice any visible signs of deterioration or if the liquid contains particles or is not clear
- Never store pen with needle still attached
- Dispose of used needles safely
- Keep in safe place, away from children
The rubber stopper is latex-free however we can not guarantee that the device has not been exposed to latex
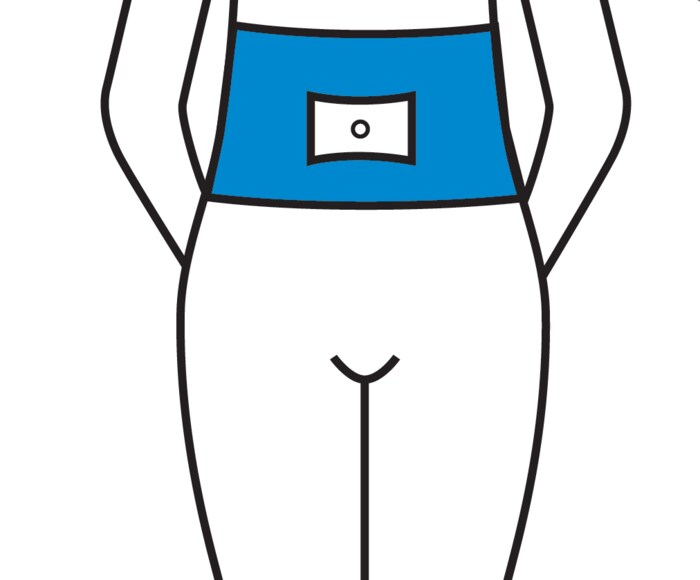
The most easily accessible area for subcutaneous injection is the abdomen. The absorption of PERGOVERIS® is the same regardless of the injection site selected. To minimize skin irritation, select a different injection site each day.
Below is a diagram with shaded areas showing the recommended subcutaneous injection sites.
If you dialed the dose passed your prescribed dose, simply turn the injection button back to the correct dose. To adjust the dose, you can turn the injection button either clockwise or counter-clockwise. After confirming the correct dose from the display window, you can proceed with the injection.
The effects of an overdose of PERGOVERIS® are unknown. You should contact your doctor for advice if you take more than your prescribed dose of PERGOVERIS®.
Do not take a double dose to make up for any doses you have missed. Contact your doctor for advice if you forget to take a dose of PERGOVERIS®.
When you cannot push the injection button down, it means your pen is empty and it is unable to provide you with your desired dose; you may still see a small amount of medication remaining in the pen – this is normal.
If the dose display shows a number higher than 0 after your injection, this indicates that the pen is empty and that the complete dose has not been given. If this happens, the number shown in the dose display indicates the missing amount of PERGOVERIS® solution that is needed to complete your dose. This is the dose you need to inject using a new pen. To complete your dose, set the dose to the missing amount (displayed on the empty pen) that you have just finished, and proceed with the injection.
You should throw away the used needles safely in a sharps container. This container is usually provided by your clinic in a PERGOVERIS® Pen Starter Kit.
You can dispose of your used PERGOVERIS® pens in an environmentally responsible manner by participating in the EMD Serono ecoProgram.
What is the ecoProgram?
EMD Serono's ecoProgram will help reduce landfill by turning used PERGOVERIS® pens to clean energy via the Waste-to-Energy (WtE) process. We care for patients, and our planet!
What is Waste-to-Energy (WtE)?
WtE is a process where waste (like used PERGOVERIS® pre-filled pens) is burned in a controlled combustion chamber at high temperatures and reduced to 10% of its original volume (hence reducing landfill). The heat generated from the combustion chambers water is turned to steam and sent through a turbine that continuously generates electricity.
According to the US Energy Recovery Council, 87 WtE plants divert approximately 90,000 tons of waste each day from landfills, generating nearly 17 billion kilowatt hours of electricity per year. This is enough to power almost two million homes and represents nearly 20% of all non-hydro renewable electricity generation in the United States. To put this figure into context, it would take 7.8 million tons of coal to produce the same amount of electricity from a coal-fired power plant. (Source: www.energyrecoverycouncil.org)
How can I participate?
First, make sure your fertility clinic is participating in the PERGOVERIS® ecoProgram. If not, ask them to join in the cause.

3 Easy steps:
1. Bring the used pens (without needles) back to your clinic.
2. Put pens in a reusable ecoProgram table-top container. When the container is full, simply empty the pens into the pre-labelled carton box provided.
3. The used pens will get sent to a WtE plant for processing to produce electricity.
Click here to understand on how to use the FRIO Mini Pocket Sharps Container.


