Learn more about
Brief Product Outline and Illustration
Brief Product Outline and Illustration
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
OVIDREL is the only recombinant human chorionic gonadotrophinhuman chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation (r-hCG). Its generic name is choriogonadotropin alfa. It is produced as a solution in a pre-filled syringe or a pre-filled pen that is intended for subcutaneous injection. OVIDREL ensures that you are receiving the highest purity hCG on the market. Subcutaneous injections offer the advantage of being convenient and easy to self-administer.
Ovidrel® is provided as a liquid in a prefilled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached and is taken by injection just under the skin (subcutaneous). With guidance, you can learn to inject yourself.
An Ovidrel® prefilled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached injection guide is included in a pamphlet called Administration and Storage Instructions, which is available for download. The guide is offered in English or French – please click on the language of your choice. Should you require injection assistance in your native language, please call the Momentum patient program at 1-800-387-8479.
Ovidrel® is provided at a dose of 250 μg as a liquid*:
250 μg/0.5 mL prefilled syringe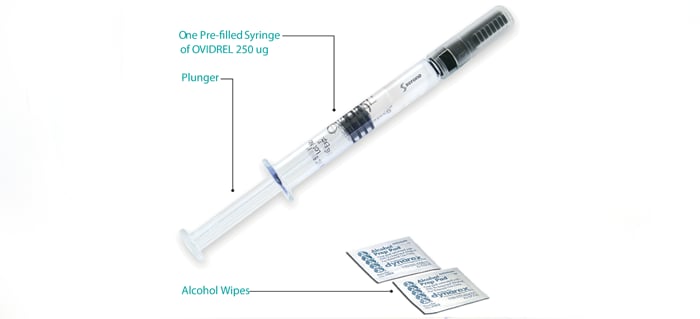
* Note that μg stands for microgram, and mL stands for milliliter, which are units of measure
Each prefilled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached should only be used once and then thrown away.
Ovidrel® is injected at a dose of 250 μg. Your doctor will recommend the appropriate treatment regimen for you.
Ovidrel® is a syntheticsyntheticmanmade or manufactured form of human chorionic gonadotropin (hCG)human chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation. When used in fertility treatments it helps folliclesfolliclessmall fluid-filled sacs in the ovary that contain the eggs released at ovulation; each month an egg develops inside the ovary in a follicle in the ovary to finish maturing and triggers ovulationovulationrelease of an egg from the ovary.
Ovidrel® is used to trigger ovulationovulationrelease of an egg from the ovary in women with ovarianovarianrelating to an ovary or the ovaries dysfunction.[1] Ovidrel® is given after treatment with drugs that stimulate follicle development.
You should not use Ovidrel® if you:
- are allergic to choriogonadotropin alpha or to any of the other ingredients of Ovidrel®
- have primary ovarianovarianrelating to an ovary or the ovaries failure
- have uncontrolled thyroidthyroida gland in the neck that secretes hormones regulating growth and development or adrenaladrenal (gland[s])adrenal (gland[s]) are small glands that sit on top of the kidneys and produce certain hormones dysfunction
- have a brain tumour in the pituitary glandpituitary glandthe gland located at the base of the brain that secretes a number of important hormones that regulate fertility, as well as normal growth and development of the body or hypothalamushypothalamusthe gland at the base of the brain that controls the release of hormones from the pituitary glands
- are pregnant
- have ovarianovarianrelating to an ovary or the ovaries cyst or enlargement of undetermined origin
- have abnormal bleeding from your uterusuterusthe womb of an unknown cause
- have ovarianovarianrelating to an ovary or the ovaries, uterineuterinerelating to the uterus (womb), or breast cancer
Please inform your doctor and pharmacist if you are taking or have taken any other medications, even those not requiring a prescription.
Contact your doctor if you experience severe pain or bloating in the stomach or pelvic area, severe upset stomach, vomiting, or weight gain, as these are symptoms of a medical event called ovarianovarianrelating to an ovary or the ovaries hyperstimulation syndrome (OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs). OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs occurs infrequently (usually less than 3% incidence). However, because OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs can progress rapidly to a serious medical event, Ovidrel® should be stopped at the first signs of OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs.
Reports of multiple births have been associated with fertility treatments. You should discuss the potential risk of multiple births with your doctor before beginning treatment.
There is no evidence to make an association between the administration of Ovidrel® to female patients and the occurrence of congenitalcongenitalpresent from birth anomalies in their children.
As with other hCGhuman chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation preparations, side effects can occur with the use of Ovidrel®: discomfort at the injection site, stomach pain, nausea, and vomiting have been reported. Ask your fertility team to discuss the possible side effects with you. As with all medications, it is important to report any physical changes and all symptoms to your healthcare professional.
Refer to the date indicated on the labels for the expiry date and not use the medication after expiry date. Store the medication in the original package, protected from light, and do not freeze Ovidrel®. Store the medication out of the reach of children.
Ovidrel® in prefilled syringes is to be stored at 2–8°C (in a refrigerator). You may store the prefilled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached at 25°C (room temperature) for up to 30 days; however, after this time the prefilled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached should be discarded.
Ovidrel® should not be used if the liquid contains particles or is not clear.
Once you have finished your injection, immediately discard the needles and syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached (without recapping the needle) into the sharps disposal container. You can take the container to a clinic or pharmacy for proper disposal.
Ovidrel® is a trademark of Merck KGaA, Darmstadt, Germany