Questions about Luveris®?
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
The effects of an overdose of Luveris® are unknown; nevertheless, there is a possibility that ovarianovarianrelating to an ovary or the ovaries hyperstimulation syndrome (OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs) may occur. If you inject more medication at one time than you were prescribed, you should contact your doctor.
In case of drug overdose, contact a health care practitioner, hospital emergency department or regional Poison Control Centre immediately, even if there are no symptoms.
If you forget to take Luveris®, do not take a double dose. In the case of a forgotten dose, please contact your doctor.
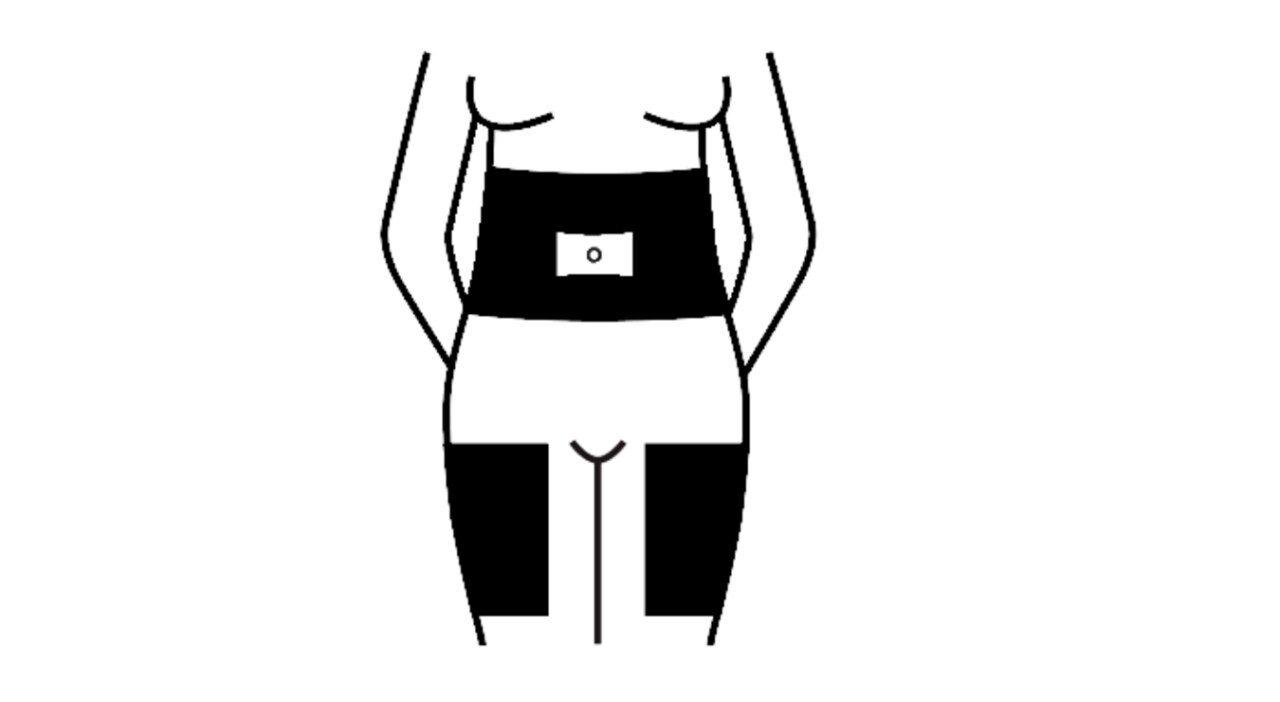
Select the site of injection (eg. top of thigh, tummy) using the injection site diagram shown here. The shaded areas show the recommended subcutaneous injectionsubcutaneous injectionadministration of medication with a fine, small needle just below the surface of the skin, into fatty tissue sites. Choose a different site each day.
Click here to understand on how to use the FRIO Mini Pocket Sharps Container