Learn more about
Brief Product Outline and Illustration
Brief Product Outline and Illustration
Call our 24/7 toll-free support line at 1-800-387-8479 or submit your questions and comments using our online form.
GONAL-f® is the brand name for the medication follitropin alpha, which is a syntheticsyntheticmanmade or manufactured version of the naturally occurring follicle stimulating hormonehormonea chemical substance produced in one part of the body that controls and regulates the activity of certain cells or organs in another part of the body (FSHfollicle-stimulating hormone (FSH)a hormone produced by the pituitary gland in the brain that stimulates the growth of follicles in the ovaries of women and sperm development in men). FSHfollicle-stimulating hormone (FSH)a hormone produced by the pituitary gland in the brain that stimulates the growth of follicles in the ovaries of women and sperm development in men is a hormonehormonea chemical substance produced in one part of the body that controls and regulates the activity of certain cells or organs in another part of the body that is produced by the pituitary glandpituitary glandthe gland located at the base of the brain that secretes a number of important hormones that regulate fertility, as well as normal growth and development of the body in the brain and in women it helps the development of eggs in the ovariesovariesthe two sexual glands of the female where the eggs are stored and that produce the hormones estrogen and progesterone.
GONAL-f® cannot be taken orally because it would be digested in the stomach and for this reason, it must be taken by injection. Due to its high level of purity, GONAL-f® is approved for subcutaneous injectiosubcutaneous injectionadministration of medication with a fine, small needle just below the surface of the skin, into fatty tissuen (just under the skin), which is easier and less painful than intramuscular injections (in the muscle). With professional guidance, you can learn to inject yourself, in the comfort and privacy of your own home. [1]
GONAL-f® injection guides are available for single dose vials within the Consumer Information part of the Product Monograph. The information is offered in English or French – please click on the language of your choice. Should you require injection assistance in your native language, please call the Momentum patient program at 1-800-387-8479.
GONAL-f® is provided as a white freeze-dried powder in a small glass container (vialviala small glass container for holding liquids) with a rubber stopper. The powder is dissolved in sterile water to make a liquid form of the medication that can then be injected. [1]
GONAL-f® is available in three different strengths*:[1]
75 IU (5.5 μg) single dose vial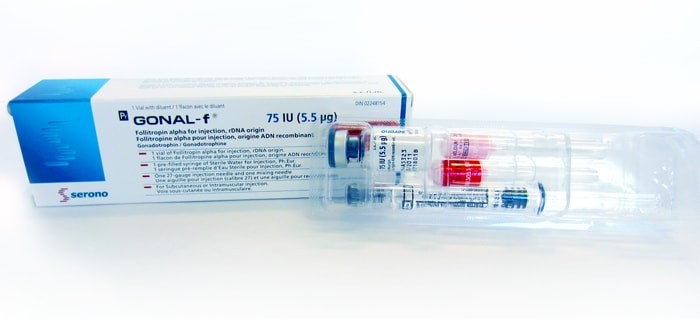
* Note that IU stands for International Units and μg stands for micrograms, which are units of measure
Each box for the single doses contains a vialviala small glass container for holding liquids of medication (white powder), a pre-filled syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached of sterile water for injection, and a needle for mixing and a needle for injection. [1]
Over the course of your treatment, doses may range between 75 IU to 450 IU, depending on your specific medical condition and your response to the medicine. Your doctor will recommend the appropriate treatment regimen for you. [1]
Folliclesfolliclessmall fluid-filled sacs in the ovary that contain the eggs released at ovulation; each month an egg develops inside the ovary in a follicle are small fluid-filled sacs in the ovary and each one contains an immature egg. GONAL-f® works by helping to stimulate the development and maturing of folliclesfolliclessmall fluid-filled sacs in the ovary that contain the eggs released at ovulation; each month an egg develops inside the ovary in a follicle and the eggs inside. [1]
GONAL-f® is used to stimulate multiple folliclesfolliclessmall fluid-filled sacs in the ovary that contain the eggs released at ovulation; each month an egg develops inside the ovary in a follicle to develop in the ovariesovariesthe two sexual glands of the female where the eggs are stored and that produce the hormones estrogen and progesterone of women undergoing fertility treatments such as in vitro fertilization (IVFin vitro fertilization (IVF)a procedure by which eggs produced by administering fertility drugs are retrieved from a woman's body and fertilized by sperm in a laboratory, and the resulting embryos are transferred by catheter to the uterus). GONAL-f® is also used to induce follicle development in some women whose menstrual cycles are either irregular or absent. In both cases, another hormonehormonea chemical substance produced in one part of the body that controls and regulates the activity of certain cells or organs in another part of the body called human chorionic gonadotropin (hCG)human chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation is also given to complete the maturing of the follicles and trigger ovulationovulationrelease of an egg from the ovary. [1]
You should not use GONAL-f® if you:
- are allergic to FSHfollicle-stimulating hormone (FSH)a hormone produced by the pituitary gland in the brain that stimulates the growth of follicles in the ovaries of women and sperm development in men or to any of the other ingredients of GONAL-f®
- have a brain tumour in the pituitary glandpituitary glandthe gland located at the base of the brain that secretes a number of important hormones that regulate fertility, as well as normal growth and development of the body or hypothalamushypothalamusthe gland at the base of the brain that controls the release of hormones from the pituitary glands
- are pregnant or breast-feeding
- have ovarianovarianrelating to an ovary or the ovaries cysts or enlarged ovariesovariesthe two sexual glands of the female where the eggs are stored and that produce the hormones estrogen and progesterone, not due to polycystic ovarianovarianrelating to an ovary or the ovaries disease (PCOpolycystic ovarian disease (PCO)the formation of cysts in the ovaries that occurs when the follicle stops developing; it is due to a hormonal imbalance in the ovary)
- have bleeding from your uterusuterusthe womb or vagina of an unknown cause
- have ovarianovarianrelating to an ovary or the ovaries, uterineuterinerelating to the uterus (womb), or breast cancer [1]
GONAL-f® is often used with other medications to stimulate ovulationovulationrelease of an egg from the ovary (e.g., human chorionic gonadotropin [hCG]human chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation, luteinizing hormone [LH]luteinizing hormone (LH)a hormone release by the pituitary gland in the brain that acts in the ovary to stimulate ripening of the follicle and formation of the corpus luteum, and clomiphene citrate), which may help the maturation of folliclesfolliclessmall fluid-filled sacs in the ovary that contain the eggs released at ovulation; each month an egg develops inside the ovary in a follicle.
A gonadotropin-releasing hormone (GnRH)gonadotropin-releasing hormone (GnRH)a hormone released from the hypothalamus in the brain that signals the pituitary gland (also in the brain) to release other hormones called luteinizing hormone and follicle-stimulating hormone (which cause follicles to develop and release a mature egg from the ovary) medication can also be given along with GONAL-f® in your treatment program. If you are also prescribed a GnRHgonadotropin-releasing hormone (GnRH)a hormone released from the hypothalamus in the brain that signals the pituitary gland (also in the brain) to release other hormones called luteinizing hormone and follicle-stimulating hormone (which cause follicles to develop and release a mature egg from the ovary) medication, your doctor may tell you to change your dose of GONAL-f®.
No other significant interactions with other medications have been reported.
As with all medication, there is a potential for side effects with GONAL-f® therapy. GONAL-f® is a syntheticsyntheticmanmade or manufactured version of follicle stimulating hormonehormonea chemical substance produced in one part of the body that controls and regulates the activity of certain cells or organs in another part of the body (FSHfollicle-stimulating hormone (FSH)a hormone produced by the pituitary gland in the brain that stimulates the growth of follicles in the ovaries of women and sperm development in men), which belongs to a group of human reproductive hormones known as gonadotropinsgonadotropins or gonadotrophinsreproductive hormones including luteinizing hormone and follicle-stimulating hormone (released by the pituitary gland) and human chorionic gonadotropin (released by the placenta).
Some women undergoing gonadotropin therapy may experience breast tenderness, fatigue, bloating, weight gain, nausea, or diarrhea. These are temporary and will resolve once treatment is stopped. Other side effects may include allergic reactions, such as a rash or local swelling at the injection site.
The greatest concern your doctor will have is ovarianovarianrelating to an ovary or the ovaries hyperstimulation syndrome (OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs). To decrease the chance of developing OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs, your doctor will carefully monitor your response to GONAL-f®. Ovarianovarianrelating to an ovary or the ovaries enlargement, sometimes accompanied by abdominal bloating and pain, may occur in about 20% of women taking gonadotropingonadotropins or gonadotrophinsreproductive hormones including luteinizing hormone and follicle-stimulating hormone (released by the pituitary gland) and human chorionic gonadotropin (released by the placenta)s. This is generally reversed by stopping treatment. Severe life-threatening cases are rare.
This is not a complete list of side effects. If you experience any unusual symptoms or side effects, you should report them to your doctor immediately.
Treatment increases your risk of developing ovarianovarianrelating to an ovary or the ovaries hyperstimulation syndrome (OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs). However, if you follow your doctor’s instructions for the recommended dose and schedule of administration, the occurrence of OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs is uncommon. GONAL-f® treatment seldom causes significant OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs unless the medicine containing hCGhuman chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation is used to induce final follicularfollicularrelating to a follicle, which is a small fluid-filled sac in the ovary that contains an egg maturing and ovulationovulationrelease of an egg from the ovary. In cases where OHSSovarian hyperstimulation syndrome (OHSS)OHSS is caused when the ovaries become overstimulated by the various hormones that cause follicular development; there is a sudden ovarian enlargement accompanied by fluid accumulation in the abdominal cavity and this may occur with or without pain, and with or without accumulation of fluid in the lungs is developing, it is recommended to withhold hCGhuman chorionic gonadotropin (hCG)a hormone produced in early pregnancy (released by the placenta after implantation of a fertilized egg) that keeps the corpus luteum producing other hormones that prevent menstruation, and women should prevent pregnancy at this time by not having sexual intercourse.
Since women with fertility problems undergoing ARTassisted reproductive technologies (ART)a variety of procedures used to bring about conception without sexual intercourse, including in vitro fertilization (IVF) and other techniques, particularly in vitro fertilization (IVFin vitro fertilization (IVF)a procedure by which eggs produced by administering fertility drugs are retrieved from a woman's body and fertilized by sperm in a laboratory, and the resulting embryos are transferred by catheter to the uterus), may have fallopian tube abnormalities, the incidence of ectopic pregnancies might be increased. Early ultrasoundultrasounda test used instead of X-rays to visualize the reproductive organs; for example, to monitor follicular development to confirm that a pregnancy is in the uterusuterusthe womb is important.
Miscarriages may be higher than in the general population, but comparable to the rates found in women with fertility problems.
There have been isolated reports of non-serious allergic reactions to GONAL-f®. If you have had this type of reaction to similar medicines, inform your doctor.
The incidence of multiple births with GONAL-f® is no different from any other gonadotropin and is dependent upon the protocol used by the clinic. Your doctor will monitor you closely to help minimize the possibility of a multiple pregnancy. The majority of births (about 80%) are single babies. Of those women who do have multiple births, the majority of these are twins. Only very few women conceive 3 or more babies. Even so, neither single nor multiple births can be guaranteed.
A causal relationship between treatment with fertility drugs and ovarianovarianrelating to an ovary or the ovaries cancer has not been established.
Store your GONAL-f® vials of powder at or below room temperature (25 °C) but do not freeze. Protect the vials from light and do not use the product after the expiry date indicated on the label.
Store your reconstituted GONAL-f® (powder has already been mixed with sterile water to form liquid in vialviala small glass container for holding liquids) as follows:
- for single-dose vials, the medication should be used immediately and any unused liquid should be thrown out with the vialviala small glass container for holding liquids
- for multi-dose vials, the liquid medication can be stored at room temperature (at or below 25°C but do not freeze). Do not store the drug in the syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached. Discard any unused liquid GONAL-f® with the vialviala small glass container for holding liquids after 28 days.
Do not use if liquid medication is cloudy, lumpy or discoloured. Keep the medication in a safe place and out of reach of children.
Once you have finished your injection, immediately discard the needles and syringesyringea hollow cylinder fitted with a sliding plunger that is used for injections when a needle is attached (without recapping the needle into a needle disposal container. Take the container to a clinic or pharmacy for proper disposal. Take the container to a clinic or pharmacy for proper disposal when the needle disposal container is full or when you have completed your entire treatment.
GONAL-f® is a trademark of Ares Trading S.A.